Since December 2020, the COVID-19 vaccines have brought hope to a fight that has spanned across the world. All three vaccines available in the United States are safe and prevent an overwhelming majority of severe and life-threatening cases of COVID-19, so they can save lives and protect our health care systems from becoming overwhelmed.
As the virus continues to mutate, it’s more important than ever for us to come together to separate fact from fiction about the COVID-19 vaccines available in the United States.
As of January 20, 2021, three COVID-19 vaccinations have been approved for Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA):
- Pfizer-BioNTech (approved for ages 12 and up)*
- Moderna (approved for ages 18 and up)
- Janssen (Johnson & Johnson) (approved for ages 18 and up)
*The Pfizer vaccine was issued an Emergency Use Authorization (EUA) by the FDA on December 11, 2020, and received full FDA approval on August 23, 2021.
Here, we’ve compiled some of the most commonly asked questions about the vaccine to help you make an informed decision that’s best for you and your family.
How was the COVID vaccine able to be developed so quickly? Is it really safe?
COVID-19 vaccines were able to be developed less than a year after the first cases were reported in Wuhan, China, thanks to a global community of experts coming together. This effort was helped by:
- Immediate publication of the genetic code of the SARS-CoV-2 virus, which allowed scientists to begin working on a vaccine before some countries had ever seen COVID-19 cases.
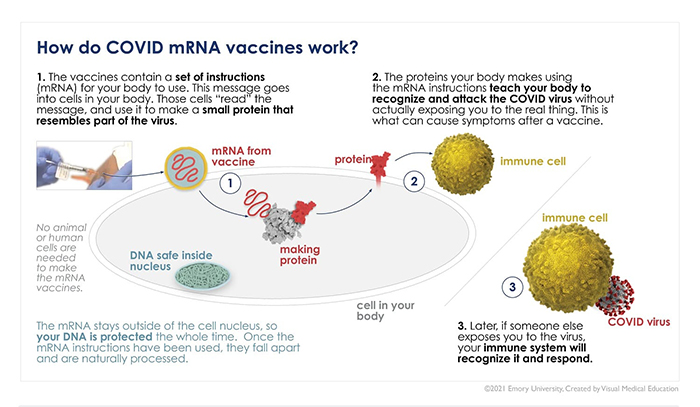
- Decades of prior research on messenger ribonucleic acid (mRNA) vaccines that scientists were able to build on to create the Pfizer and Moderna vaccines.
- Many volunteers, including some right here at Emory, who were willing to help test the vaccine.
- Support from government programs and private funding.
While the vaccines were developed quickly, they still adhered to stringent safety protocols, including:
- Closely regulated and monitored clinical trials to ensure the vaccines were safe without serious side effects.
- Development and implementation of vaccine safety monitoring to ensure serious side effects aren’t present in the general population over time.
Why should I take the vaccine if the COVID mortality rate is relatively low?
From January 2020 to September 20, 2021, there have been at least 675,071 deaths caused by COVID-19. Many experts believe this number is likely significantly higher, largely due to limited early testing and confusion about how to record deaths in the beginning of the pandemic. As a comparison, the CDC estimates that there were approximately 22,000 deaths from influenza (flu) during the 2019-2020 season.
Currently, the mortality rate of COVID-19 in the United States is 1.6%, according to the Johns Hopkins Coronavirus Resource Center. With so many people infected, that has meant hundreds of thousands of deaths, including people who were our family, friends, and neighbors. If everyone in the United States were infected with COVID-19, a 1.6% mortality rate could lead to as many as 5 million deaths.
Additionally, even if your mortality risk from COVID-19 is lower because of your age or health status, you could still spread the virus to someone more vulnerable – for example, a parent or grandparent, or someone immunocompromised – who might be at high risk for complications and death.
Finally, recent studies estimate that between a quarter and a third of all people infected with COVID have long-term symptoms. So even if you aren’t at significant risk of dying, a COVID infection could still leave you with debilitating symptoms that prevent you from working or doing daily activities that you enjoy.
The vaccine and continued public health efforts (mask wearing, physical distancing, handwashing) all help to slow the spread of the virus and prevent deaths.